Epigenetic modulators link mitochondrial redox homeostasis to cardiac function
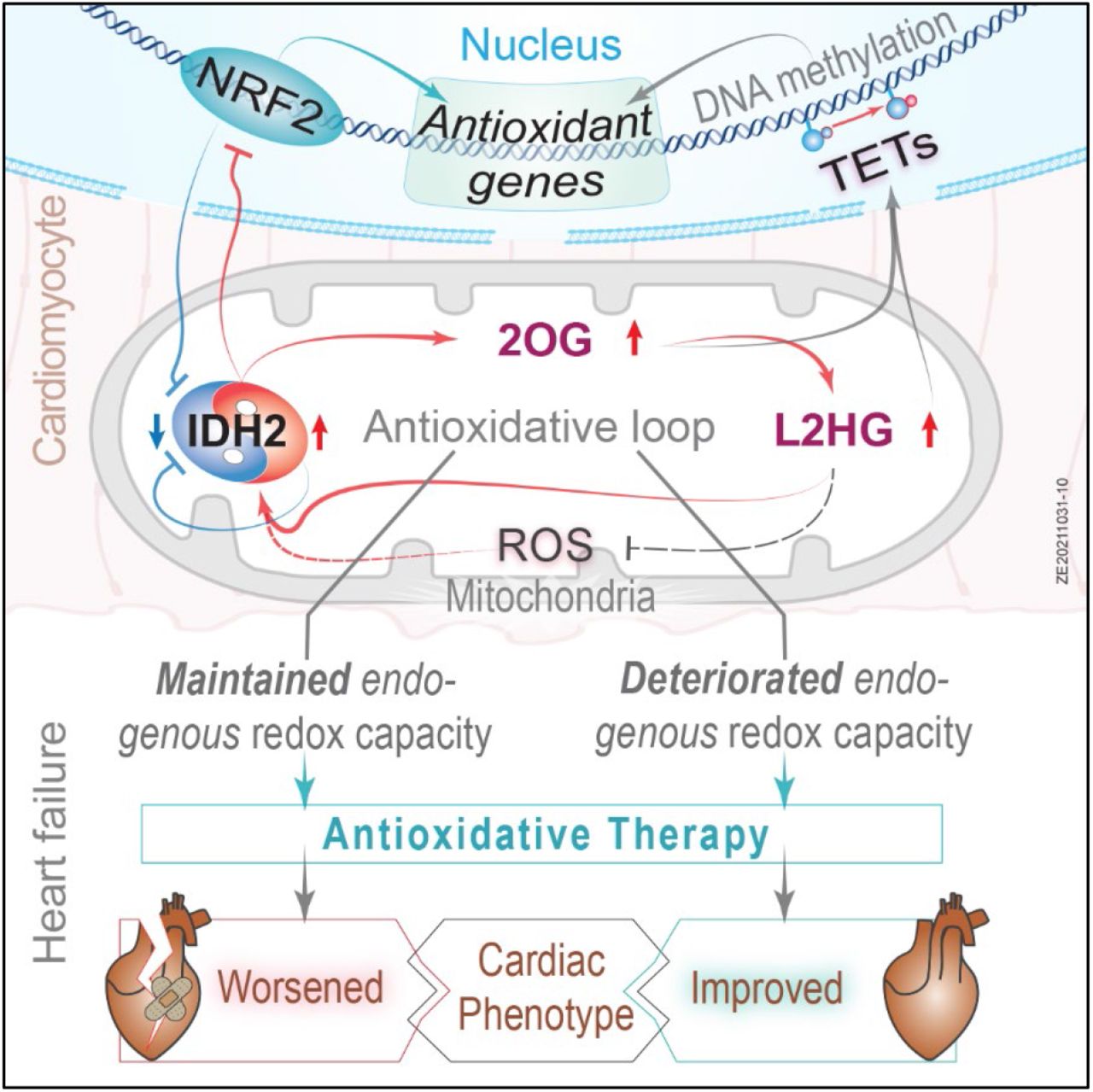
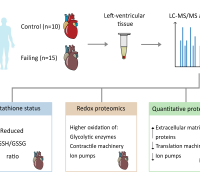
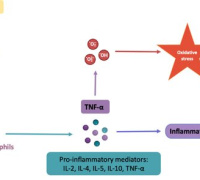
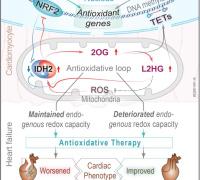
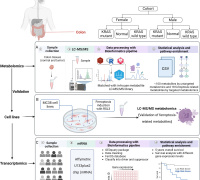
In a groundbreaking revelation, Elbeck et al, have uncovered a critical defense mechanism against reactive oxygen species (ROS) production, a hallmark of numerous diseases. Contrary to previous studies overlooking the role of endogenous antioxidative defenses, a pioneering multi-omics approach has showcased the pivotal role of mitochondrial isocitrate dehydrogenase (IDH2) in cardiomyocytes as a major antioxidant defense.
Surprisingly, both male and female mice and humans experiencing heart failure exhibit a paradoxical reduction in the expression of IDH2. However, this reduction is compensated by a remarkable increase in the enzyme's activity, revealing an intricate adaptation mechanism in the face of cardiac challenges.
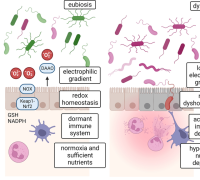
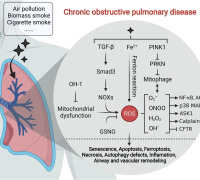
The research team's findings extend further, describing an elaborate mutual regulation between the antioxidant activities of IDH2 and NRF2. This regulatory network is intricately woven through a system involving 2-oxoglutarate and L2-hydroxyglutarate, with additional mediation through unconventional hydroxymethylation of cytosine residues found in introns.
In a pivotal development, the conditional targeting of ROS in a murine model of heart failure has demonstrated a significant improvement in cardiac function.
In summary:
- Paradoxical downregulation of mitochondrial isocitrate dehydrogenase (IDH2) in response to oxidative stress leads to the discovery of a robust antioxidative defense in the heart.
- An antioxidative loop involving IDH2 coordinates other antioxidative defenses, such as NRF2.
- This loop produces epigenetic modifications that link oxidative stress to mitochondrial function.
- The conclusion that enhancing antioxidative capacity improves cardiac function only when the endogenous capacity is insufficient opens new approaches to individualized treatment of patients with heart failure.
These results challenge conventional wisdom, providing a potential explanation for the historical shortcomings of antioxidant-based treatments for heart failure.
This groundbreaking research not only uncovers a previously overlooked aspect of cardiac biology but also opens new avenues for personalized approaches in treating heart failure. These insights mark a paradigm shift, offering hope for more effective and tailored treatments that could revolutionize the management of heart health.
Photo credits: Elbeck et al. (2023)