Advanced Redox and Protein Profiling of Failing Human Hearts Using Mass Spectrometry
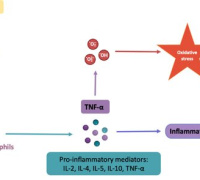
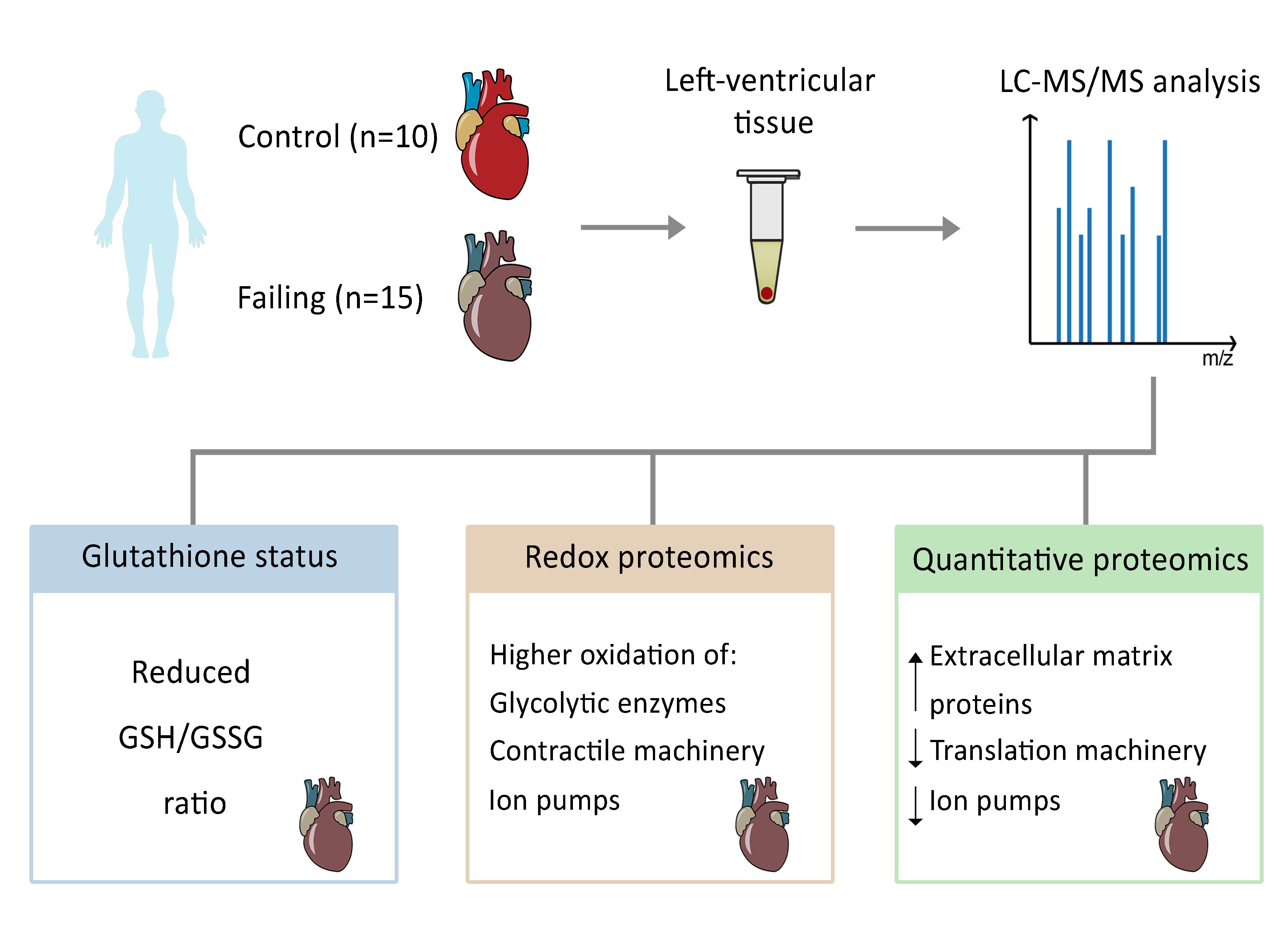 Tomin et al. (Vienna University of Technology) have conducted a groundbreaking study using mass spectrometry to analyze the redox state and protein profiles of failing human hearts. Oxidative stress, a key factor in heart failure, damages the myocardium, impairs antioxidant defenses, disrupts redox signaling, and harms proteins.
Tomin et al. (Vienna University of Technology) have conducted a groundbreaking study using mass spectrometry to analyze the redox state and protein profiles of failing human hearts. Oxidative stress, a key factor in heart failure, damages the myocardium, impairs antioxidant defenses, disrupts redox signaling, and harms proteins.
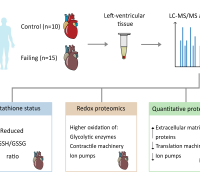
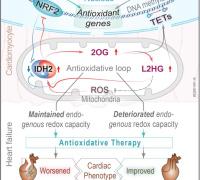
The study involved comprehensive analyses of left-ventricular tissue from both healthy and failing human hearts. The findings revealed that failing hearts have lower ratios of glutathione to glutathione disulfide and increased oxidation of various proteins, including those involved in contraction and glycolysis. Additionally, quantitative proteomics showed an increased presence of proteins linked to extracellular matrix remodeling and a decrease in several ion transporters, aligning with observed contractile impairment.
These effects were also replicated in an in vitro cell culture model under controlled oxygen conditions. This study is the most extensive to date, integrating analyses of protein abundance, redox state, and global proteome profiles in end-stage failing human hearts and cultured human cardiomyocytes.