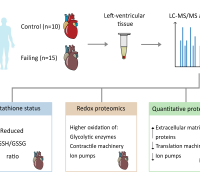
Redox Nanomedicine: Towards Ameliorating Chronic Kidney Disease
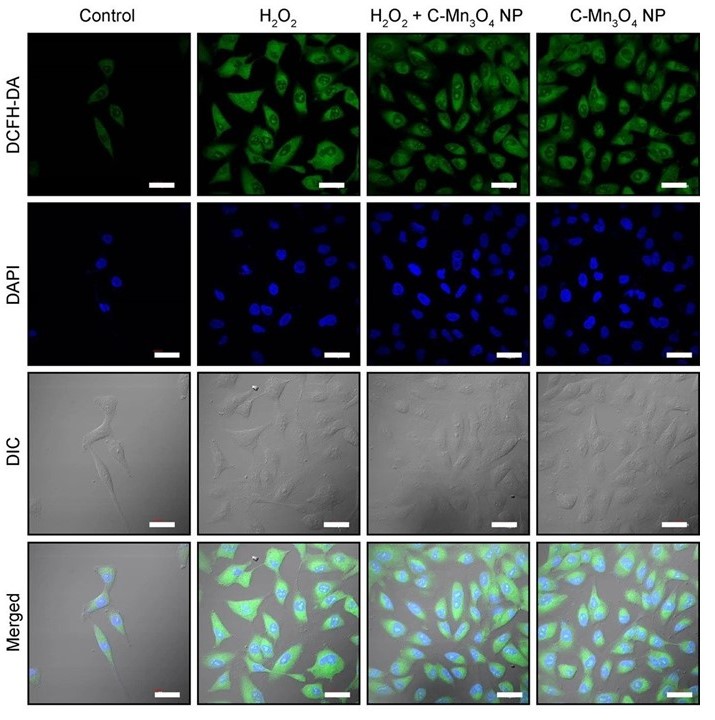
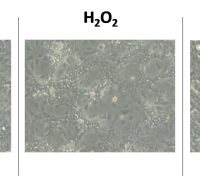
Confocal fluorescence micrographs of HEK 293 cells stained with DCFH2-DA and counterstained with DAPI. Cells were either left untreated or pretreated with C-Mn3O4 NPs (30 μg mL−1) prior exposure to H2O2 (100 µM).
Oxidative stress plays a major role in renal damage. It is characterized by increased intracellular levels of reactive oxygen species (ROS), which are linked with tissue damage, inflammation and increased risk of degenerative diseases.
Targeting reactive oxygen species (ROS) while maintaining cellular redox signaling is crucial in the development of redox medicine as the origin of several prevailing diseases including chronic kidney disease (CKD) is linked to ROS imbalance and associated mitochondrial dysfunction.
It has been previously suggested that metal oxide nanoparticles with electron-donating and accepting potential exhibit antioxidant activity by preventing the damage caused by free radicals to cells. Thus, Adhikari et al. explored the therapeutic potential of citrate-functionalized manganese oxide (Mn3O4) nanoparticles (C‑Mn3O4 NPs), knowing that C‑Mn3O4NP complex can be attributed to its role in redox regulation and prevention of mitochondrial damage.
They reported that a potential nanomedicine comprising of Mn3O4 nanoparticles duly functionalized with biocompatible ligand citrate (C-Mn3O4 NPs) can maintain cellular redox balance in an animal model of oxidative injury.
- They developed a cisplatin-induced CKD model in C57BL/6j mice with severe mitochondrial dysfunction and oxidative distress leading to the pathogenesis. Four weeks of treatment with C-Mn3O4 NPs restored renal function, preserved normal kidney architecture, ameliorated overexpression of pro-inflammatory cytokines, and arrested glomerulosclerosis and interstitial fibrosis.
- A detailed study involving human embryonic kidney (HEK 293) cells and isolated mitochondria from experimental animals revealed that the molecular mechanism behind the pharmacological action of the nanomedicine involves protection of structural and functional integrity of mitochondria from oxidative damage, subsequent reduction in intracellular ROS, and maintenance of cellular redox homeostasis.
Successful clinical translation of this nanomedicine may open a new avenue in redox-mediated therapeutics of several other diseases where oxidative distress plays a central role in pathogenesis.
© Image- Adhikari et al. Commun Biol 4, 1013 (2021)
Redox Medicine 2023 will dedicate a whole session to "Redox Medicine & Diseases: Mechanism of Action & Strategies". You can share your latest research under this session by submitting your abstract.
Know more about this year's sessions.
Media Contact:
Redox Medicine Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
Redox Medicine 2023 Congress
June 21-23, 2023 - Paris, France
Website | LinkedIn | Facebook