Research challenges “sugar hypothesis” of diabetic cataract development
New findings from investigators at Brigham and Women’s Hospital, a founding member of the Mass General Brigham healthcare system, contradict previous notions about sugar’s role in the onset of diabetic cataracts. Using an animal model that more closely recapitulates type 2 diabetes in humans, the research team found early signs of damage in the eye before the onset of type 2 diabetes, suggesting that diabetic complications may start during the pre-diabetic state. Results are published in the Journal of Biomedical Science.
A pitfall in the scientific process is not that a theory could be false, but that it is taken for granted when it seems logical and not challenged with new experiments,” said Ali Hafezi-Moghadam, MD, PhD, director of the Molecular Biomarkers Nano-Imaging Laboratory (MBNI) in the Department of Radiology at the Brigham and an associate professor at Harvard Medical School. “The evidence we have collected here calls the long-believed theory about diabetic cataracts into question, begging us to reexamine the current dogma that has been relied on for explaining diabetes-associated cataracts.”
Cataracts—the clouding of the lens of the eye—are the number one cause of blindness worldwide and are a common complication of type 2 diabetes. The current hypothesis behind diabetic cataract development is coined “the sugar hypothesis” and suggests that high blood sugar—a hallmark of diabetes—precedes cataract development. The working assumptions underlying the sugar hypothesis describe higher levels of glucose in the lenses of people with diabetes convert to a sugar alcohol molecule called sorbitol, which induces structural changes to the lens of the eye that precede cataract development. While unproven, researchers rarely investigate this theory further due to cataracts’ treatable nature.
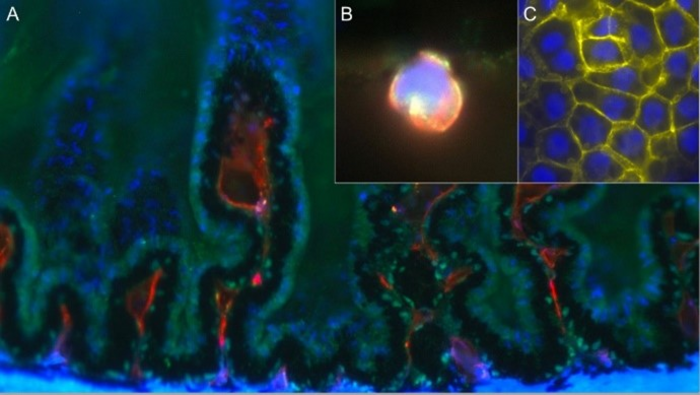
Hafezi-Moghadam and colleagues studied the Nile grass rat, a model that they originally reported spontaneously develops type 2 diabetes when kept in captivity and closely mimics the condition in humans. Much like humans with type 2 diabetes, these animals first develop insulin resistance and high blood insulin levels before their blood glucose rises above normal. Using a specialized technique termed stereo microscopy with dual bright-field illumination, researchers observed the development of dot-like microlesions, which predisposed cataract formation, in the inner cortical regions of the lens. Unexpectedly, they noticed that in nearly half of the animals tested, these microlesions appeared before the animals developed hyperglycemia, or high blood sugar, suggesting that it was not raised blood glucose levels themselves leading to cataract development.
Instead, researchers identified that immune cells were migrating from specialized structures in the eye called the ciliary bodies toward the lens. In these areas, where the immune cells traversed the capsule of the lens, they found that the epithelial cells that normally cover the inner surface of the lens capsule changed their identity and behaved differently. These changes, also referred to as epithelial-mesenchymal transformation (EMT), were followed by seemingly unorganized cell growth, cell death, and cell migrations into the body of the lens. In some regions, the newly transformed cells simply vacated their original positions and made their way into the lens. Such cellular changes, however small in dimensions, significantly compromise the function of the lens.
While still too early to tell what exactly causes the immune cells and epithelial cells to behave the way they do, the researchers conclude that their study urges further investigation of prevailing theories. It may also bring the medical community a step closer to understanding the cellular mechanisms underlying the origins of diabetic complications during the pre-diabetic stage of the disease. And once we understand the pathogenesis, Hafezi-Moghadam envisions, we can start to search for how to prevent people with diabetes from developing cataracts and potentially other complications elsewhere in the body.
“While cataracts today are easily removable with surgery, this procedure comes with the risk of complications and is expensive, both for individuals and our healthcare system,” said Hafezi-Moghadam. “With over 500 million people worldwide and 37 million Americans having diabetes, the great majority of whom have type 2, there is an incentive for trying to find non-surgical ways of preventing, slowing, or even reversing this complication. Perhaps one day it will become possible to avoid performing these surgeries altogether. And that requires that we return to the basics of the cellular processes underlying cataract development.”
Funding: This work was supported by NIH Impact Award (DK108238-01, AHM), and JDRF
Innovation Award (INO-2016-222-A-N, AHM).
Paper cited: Hafezi-Moghadam et al. “Pre-hyperglycemia immune cell trafficking underlies subclinical diabetic cataractogenesis.” Journal of Biomedical Sciences DOI: 10.1186/s12929-023-00895-6
News source: www.eurekalert.org
Image Credit: Images provided by Hafezi-Moghadam et al.
Media Contact:
Redox Medicine Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
Redox Medicine 2023 Congress
June 21-23, 2023 - Paris, France
Website | LinkedIn | Facebook